Chemical Analysis of Water
Estimation of free chlorine #
Alkalinity #
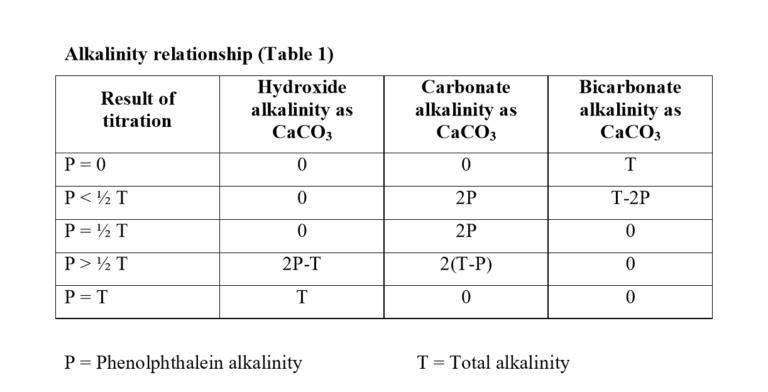
Alkalinity is present due to OH⁻, CO₃⁻², HCO₃⁻
- OH⁻ and HCO₃⁻ do not exist together
- If one is present the other can’t be: OH⁻+HCO₃⁻=CO₃²⁻+H₂O
- OH⁻
- CO₃²⁻ [these two consist of P, for Phenolphthalein indicator]
- HCO₃⁻ [the above three consist of M, for Methyl orange indicator]
Reactions: OH⁻+H⁺ → H₂O CO₃²⁻+H⁺ → HCO₃⁻ HCO₃⁻+H⁺ → H₂O+CO₂
Determination of Alkalinity of water https://readcivil.com/determination-alkalinity-water/
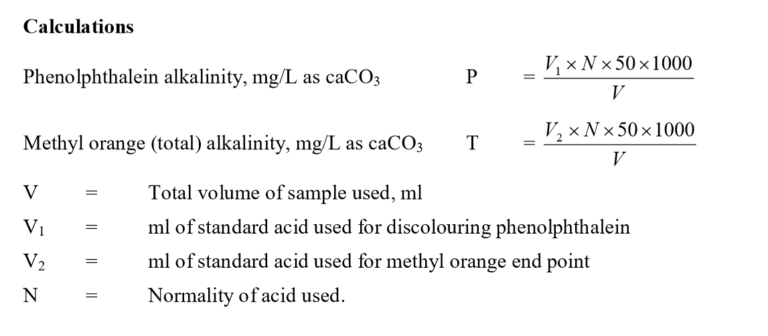
- Pipette 20 mL of sample into a clean Erlenmeyer flask (V ml).
- Add two drops of phenolphthalein indicator. If the pH is above 8.3 the colour is pink.
- Titrate this sample against standard acid, 0.02 N H2SO4 in the burette till the colour just disappears. Note the volume of the titrant used (V1 ml).
- Then add two drops of methyl orange indicator. The colour turns yellow.
- Titrate this again against the acid in the burette, till the yellow colour just turns orange-yellow.
- Note the volume of titrant used (V2 ml)